New National Water Quality Standard! HJ 1395-2024 is Here: A Guide to Measuring 17 Heterocyclic Pesticides
- On
- InGuide
On December 25, 2024, the Ministry of Ecology and Environment officially released the national standard “HJ 1395-2024 Water Quality—Determination of 17 Heterocyclic Pesticides.” Drafted under the leadership of the Environmental Monitoring Stations of Hunan and Sichuan provinces and jointly validated by six authoritative testing institutions including the National Center for Analysis and Testing, this standard will be formally implemented starting July 1, 2025. It provides a unified technical basis for the accurate detection of heterocyclic pesticides in water quality.

I. Detection Principle: Deconstruction of Core Steps
The 17 heterocyclic pesticides in a sample first undergo pre-treatment via liquid-liquid extraction (LLE) or solid-phase extraction (SPE). They are then separated and detected using a High-Performance Liquid Chromatograph (HPLC) equipped with an Ultraviolet (UV) detector or a Diode Array Detector (DAD). Identification is based on retention time, and quantification is performed using the external standard method, ensuring the accuracy and reliability of the detection results.
The following table lists the 17 heterocyclic pesticides covered by the standard:
| No. | CAS No. | Compound Name |
|---|---|---|
| 1 | 138261-41-3 | Imidacloprid |
| 2 | 1912-24-9 | Atrazine |
| 3 | 67747-09-5 | Prochloraz |
| 4 | 6190-65-4 | Atrazine-desethyl |
| 5 | 120068-37-3 | Fipronil |
| 6 | 10605-21-7 | Carbendazim |
| 7 | 135410-20-7 | Acetamiprid |
| 8 | 43121-43-3 | Triadimefon |
| 9 | 1007-28-9 | Atrazine-deisopropyl |
| 10 | 21087-64-9 | Metribuzin |
| 11 | 7287-19-6 | Prometryn |
| 12 | 2163-68-0 | 2-Hydroxyatrazine |
| 13 | 21725-46-2 | Cyanazine |
| 14 | 32809-16-8 | Procymidone |
| 15 | 55219-65-3 | Triadimenol |
| 16 | 122-34-9 | Simazine |
| 17 | 70258-18-3 | 2-Chloro-5-chloromethylpyridine |
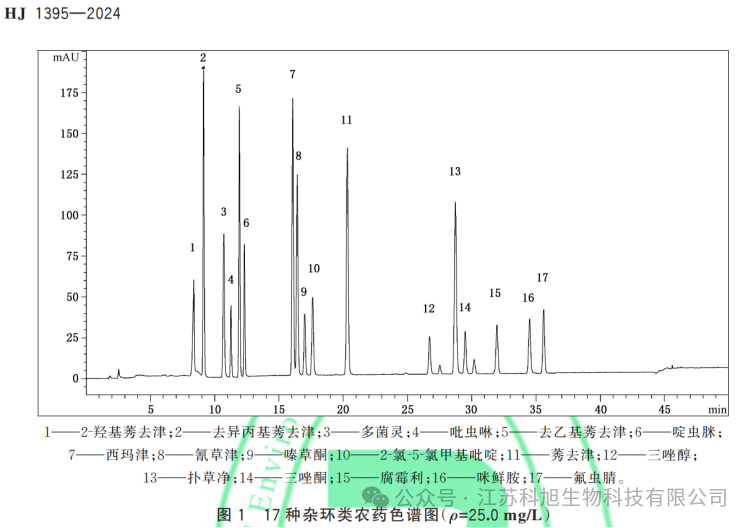
The following figure shows the chromatogram of the 17 heterocyclic pesticides covered by the standard:

II. Complete Experimental Process: From Sampling to Detection
2.1 Sampling and Preservation: Controlling the “First Gate”
Sampling must strictly adhere to regulations specified in standards such as GB 17378.3, HJ 91.1, HJ 91.2, HJ 164, and HJ 442.3 to ensure sample representativeness. After collection, the sample pH must be immediately adjusted to a range of 4–8 using hydrochloric acid or sodium hydroxide solution. The sample must then be refrigerated below 4°C and protected from light to prevent degradation of pesticide components.

2.2 Sample Preparation: Selection Among 3 Pre-treatment Methods
Depending on sample characteristics (e.g., salinity, suspended solids content, and stability of target compounds), one of the following three pre-treatment methods can be chosen:
① Direct Injection (Simple and Efficient)
Applicable Scenarios: Clean samples with low salinity and low suspended solids.
Procedure: Mix the sample → Filter through a 0.45 μm regenerated cellulose membrane → Discard the first 1 mL of filtrate → Transfer 1 mL of the filtrate to an injection vial for analysis.
② Liquid-Liquid Extraction (LLE) (High Recovery Rate)
Applicable Scenarios: Samples with complex matrices.
Procedure:
a. Measure a sample volume, adjust pH to 7–9 with sodium hydroxide, add sodium chloride and shake well.
b. Extract three times with dichloromethane, shake for 5 min each time, allow phases to separate.
c. Pass the organic phase through anhydrous sodium sulfate to remove water, combine the extracts, and concentrate to 1 mL.
d. Choose the appropriate silica gel cartridge cleanup method based on sample characteristics and compound properties.
③ Solid-Phase Extraction (SPE) (Efficient Cleanup)
Applicable Scenarios: Trace pesticide detection, low-contamination samples.
Procedure:
a. Condition the SPE cartridge with 10 mL of methanol followed by 10 mL of water.
b. Pass the sample through the cartridge at a flow rate of 5 mL/min. Rinse the sample bottle twice with water and pass the rinsate through the cartridge.
c. Wash the cartridge (discard washings) and dry it under vacuum.
d. Elute with methanol at a flow rate of 1–2 mL/min. Collect the eluate, concentrate, and dilute to 1.0 mL for analysis.
2.3 Instrumental Analysis: Parameter Setup, Identification and Quantification
① Instrument Parameter Setup
Injection Volume: Adjust based on the pre-treatment method used (direct injection vs. extraction methods differ).
Mobile Phase and Elution Gradient: Select according to instrument type (binary/ternary pump). Gradient elution programs can be appropriately optimized.
Detection Wavelength: Set according to the maximum UV absorption wavelength for each compound to avoid interference.
② Standard Curve Preparation
Direct Injection Method: Concentration range 0.02–5.00 mg/L.
LLE / SPE Methods: Concentration range 0.100–25.0 mg/L.
③ Identification and Quantification
Identification: Primarily based on the retention time of the target compound. Supplementary methods such as “standard addition, signal ratio at different wavelengths, UV spectrum scanning, or MS verification” may be used.
Quantification: Calculate using the formula corresponding to the chosen pre-treatment method. Report results with three significant figures; the number of decimal places should match the method detection limit.
III. Experimental Challenges & Key Considerations: A Guide to Avoiding Pitfalls
3.1 Sampling Stage: Pay Attention to Holding Times!
Holding times are strictly limited due to varying stability among different pesticides:
2-Chloro-5-chloromethylpyridine: Only 24 hours.
Procymidone: Only 2 days.
The other 15 pesticides: Maximum 7 days.
3.2 Sample Preparation: Don’t Overlook These Details
① Direct Injection Method Precautions
Not Applicable: For samples with high salinity (e.g., seawater) or for prochloraz determination when suspended solids > 75 mg/L.
② Key Control Points for Liquid-Liquid Extraction
Extraction Time: Procymidone degrades easily at room temperature; control time within 5–10 minutes.
Concentration Rate: Too fast leads to low recovery of “2-Chloro-5-chloromethylpyridine”; too slow leads to low recovery of “Procymidone”.
③ Core Requirement for Solid-Phase Extraction
Keep the column bed wet at all times! If the column bed dries before sample loading, recovery of some compounds may drop significantly.

3.3 Instrumental Analysis Stage: Parameter Matching is Key
Due to different pre-treatment methods, the concentration of the final test solution varies greatly. Adjust the “injection volume, standard curve concentration, and calculation method” accordingly.
Detection at low wavelengths is prone to interference. Carefully check the wavelength requirements for each compound as specified in the standard.
3.4 Data Processing: Identification Pitfalls to Avoid
Retention times of the 17 compounds may overlap. It is recommended to:
First locate peaks using single-compound standard solutions, then analyze mixed standard solutions.
When conditions permit, prioritize using mass spectrometry (MS) for辅助定性 to improve accuracy.
IV. Summary
The implementation of HJ 1395-2024 will further standardize the detection process for heterocyclic pesticides in water, providing more precise technical support for water environmental supervision. During the experimental process, close attention must be paid to four major aspects: “sample holding times, pre-treatment details, instrument parameter matching, and identification method verification” to efficiently achieve accurate determination of the 17 pesticides.
This standard will be formally implemented starting July 1, 2025. Relevant testing institutions can use this time to familiarize themselves with the procedures, optimize their methods, and prepare for implementation. To support the implementation of this standard, Jiangsu Kexu Biotechnology Co., Ltd. has prepared all relevant reference materials. Inquiries are welcome.
V. Contact Us
Phone: Mr. Liu 13776846735, Mr. Gu 15851932062, 0519-89988589
📍 Address: No. 8 Changxiu Road, Economic Development Zone, Wujin District, Changzhou, Jiangsu Province, China
Email: justin.liu@bioguidelab.com, biokoxu@bioguidelab.com
